Abstract
Abstract Background: COVID19 in patients affected by lymphoma represents an important challenge because of the higher mortality rate compared to the general population, due to the underlying disease and the immunosuppressive treatments that make them a particularly vulnerable population. Anti-SARS-CoV-2 monoclonal antibodies (anti-S MoAbs) appear promising in this setting.
Methods: We report a monocentric retrospective study including 176 patients with a diagnosis of lymphoma who developed SARS-CoV-2 infection and consecutively observed since the start of COVID19 pandemic (March 2020-March 2022). Patients were compared in terms of infection outcome according to lymphoma-related and COVID-related risk factors.
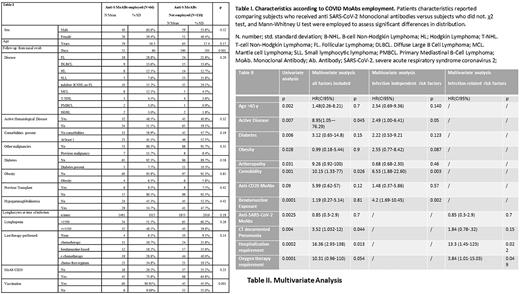
Results: Median days of follow-up at June 15, 2022 were 106 (range 67-788); median age was 61.5 years (SD 17.09) (range 22-99), 56.8% (n=100) were male and 43.2% (n=76) were female. Forty-four (26%) patients had aggressive non-Hodgkin lymphoma (NHL), including 13 mantle cell lymphoma, 103 (66%) had indolent NHL, including 43 follicular lymphoma, 7 (4%) had T-Cell Lymphoma, and 22 (13.2%) had Hodgkin lymphoma. Patients characteristics according to Anti S-MoAbs employment are reported in Table I. Overall, mortality rate was 13.1%, with a decreasing trend between first waves to the last wave of pandemic (18.5% vs 9.4%, p 0.076). Patients receiving Anti-S MoAbs were 41.3% (n=66), of which 42 received sotrovimab,18 bamlanivimab/etesevimab and 6 casirivimab/imdevimab. Anti-S MoAbs treated patients showed inferior mortality rate (overall survival, OS 93.2% vs 82.7%, p 0.025,Table II) with no serious toxicity, reduced documented pneumonia (26% vs 33%, p 0.005), and reduced need of oxygen support (14.5% vs 35.7%, p 0.003). Among patients who received 3 doses of vaccine, the employment of Anti-S MoAbs showed a trend of superior survival versus those who did not receive Anti-S MoAbs (OS rates 97.3% vs 84.2%, p 0.064). Death rate was 10.5% among vaccinated patients and 13.6% for non-vaccinated (p 0.54). Patients receiving at least one dose of vaccine (64%) showed a significantly lower incidence of fever (61.4% vs 84.2%, OR 0.69 range 0.56-0.85, p 0.002), of manifest symptoms (79.2% vs 93%, OR 0.71 range 0.57-0.91, p 0.017), of CT-documented pneumonia (29% vs 41%, p 0.04) and of oxygen support requirement (19.2% vs 39.7%, OR 0.65 range 0.45-0.92, p 0.005). Univariate analysis was performed to assess the death risk associated to patient-related, lymphoma-related or infection-related features. Factors showing a significant association with death are reported in Table II. Among patient-related risk factors, age above 65, having at least 1 comorbidity, diabetes and obesity, resulted significantly associated with an unfavorable outcome. Among lymphoma-related risk factors, previous exposure to bendamustine (OS 64%, 10/29 vs 91% 13/147, p 0.0001) and active disease or ongoing onco-hematological treatment at the time of infection (OS 77% vs 92% p 0.002) resulted significantly associated with worse outcome (Table II); bendamustine treatment increased death-risk independently from anti-CD20 exposure. Patients with progression of lymphoma during COVID19 infection and those who delayed the onco-hematological treatment for the infection had a significantly lower OS, 60% (p 0.001) and 80% (p 0.002), respectively. Regarding COVID19 severity factors, the presence of cough, documented pneumonia, hospitalization requirement (OS 59% 34/58, vs 97% 103/106, p 0.0001) and oxygen support therapy (OS 56%, 25/44 vs 97%, 112/116, p 0.0002) (Table II) were strongly associated with impaired survival. On multivariate analysis, active hematological disease (OS 72% (HR 2.49 CI1.00-6.41), bendamustine exposure (OS 60% HR 4.2 CI 1.69-10.45) and at least one comorbidity (HR 6.53 CI 1.88-22.60) proved independent prognostic factors for death risk (Table II). Multivariate analysis including only features of COVID 19 severity showed that oxygen therapy and hospitalization had the highest impact on infection outcome (Table II). Conclusion: Our findings encourage the use of anti-S MoAbs in the management of COVID19 in lymphoma patients to mitigate the mortality rate, and demonstrate the increased mortality in patients treated with bendamustine-based regimens and in those with active hematologic disease at the time of infection.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal